Stay Ahead with Shein Chung Chow’s Cutting-Edge Designs
Count on our Shein Chung Chow range for reliability and premium quality.
Handbook of Adaptive Designs in Pharmaceutical and Clinical Development
Sample Size Calculations in Clinical Research (Chapman & Hall/CRC Biostatistics Series)
Shein-Chung ChowDesign and Analysis of Bioavailability and Bioequivalence Studies, Second Edition (Chapman & Hall/CRC Biostatistics Series)
Biosimilar Drug Product Development
Statistics in Drug Research: Methodologies and Recent Developments
Quantitative Methods for HIV/AIDS Research
Statistical Design and Analysis in Pharmaceutical Science: Validation, Process Controls, and Stability (STATISTICS, A SERIES OF TEXTBOOKS AND MONOGRAPHS) Hardcover – Import, 22 February 1995
Sample Size Calculations in Clinical Research, Second Edition (Chapman & Hall/CRC Biostatistics Series)
[(Encyclopedia of Biopharmaceutical Statistics)] [ Edited by Shein-Chung Chow ] [May, 2010]
Analytical Similarity Assessment in Biosimilar Product Development
Controversial Statistical Issues in Clinical Trials: 42 (Chapman & Hall/CRC Biostatistics Series)
Statistics in Drug Research: Methodologies and Recent Developments: 10 (Biostatistics, 10)
Meta-analysis for Public Health and Medical Research (Chapman & Hall/CRC Biostatistics Series)
Mark ChangAdaptive Design Methods in Clinical Trials (Chapman & Hall/CRC Biostatistics Series)
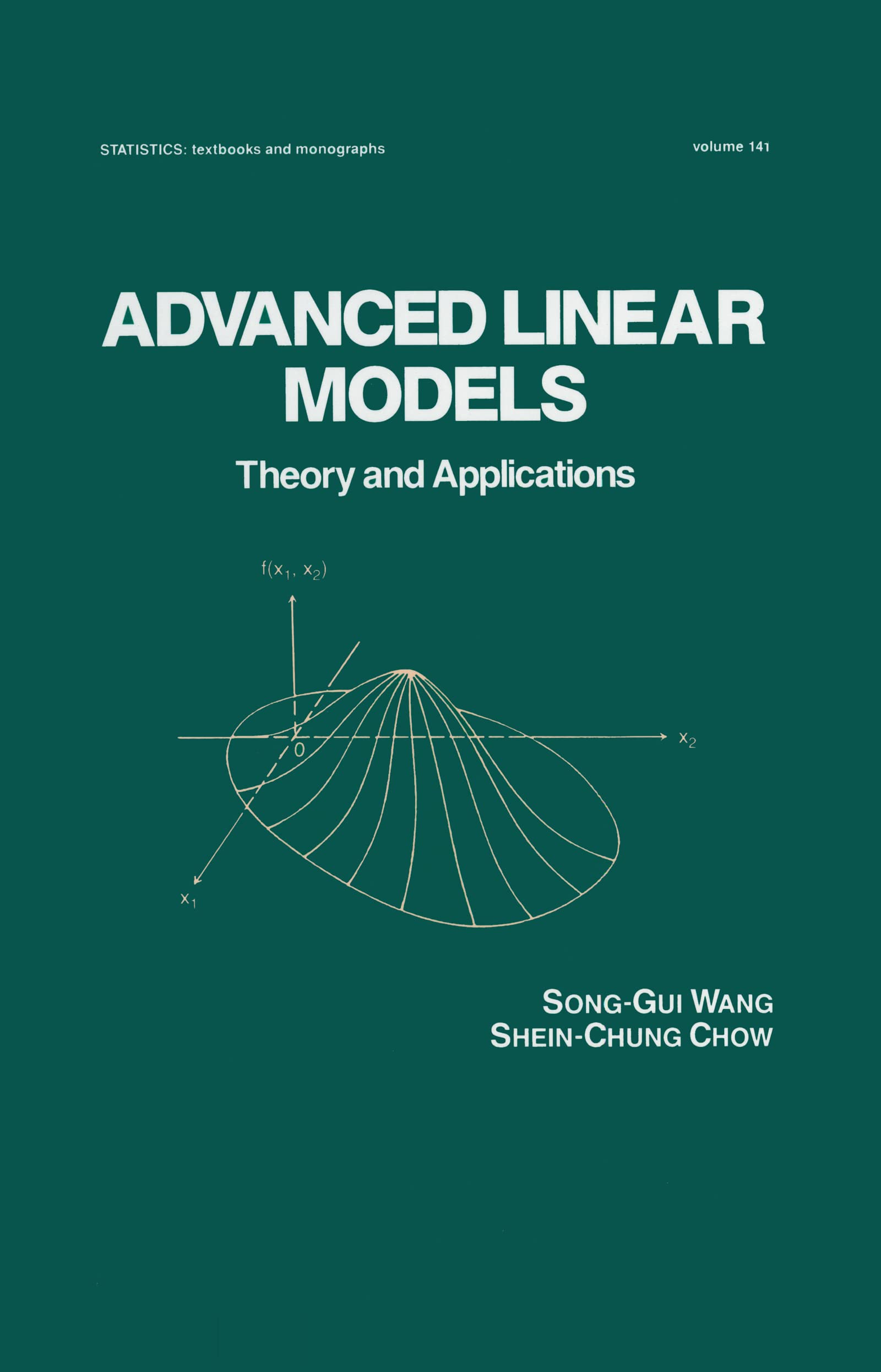
Advanced Linear Models: Theory and Applications
UMAI Chung Jung One Korean Dang Myun Glass Noodles 2.4 Kg
Statistical Design and Analysis of Stability Studies: 19 (Chapman & Hall/CRC Biostatistics Series)
Statistics in Drug Research: Methodologies and Recent Developments
中医药定量研究方法,原著 周贤忠(Shein-Chung Chow),上海科学技术出版社【质量保障 放心购买】
Quantitative Methods for Traditional Chinese Medicine Development
Design and Analysis of Clinical Trials: Concepts and Methodologies
Design and Analysis of Clinical Trials: Concepts and Methodologies (Wiley Series in Probability and Statistics) Hardcover – 6 Jan. 2004
Statistics in Drug Research: Methodologies and Recent Developments
Design and Analysis of Bioavailability and Bioequivalence Studies
Adaptive Design Methods in Clinical Trials
Design and Analysis of Bridging Studies (Chapman & Hall/CRC Biostatistics Series)
Randomized Clinical Trials of Nonpharmacological Treatments
Sample Size Calculations in Clinical Research
Chung -Shi Dux Ortho, Unisex Adults' Clogs
Advanced Statistics in Regulatory Critical Clinical Initiatives
Statistical Design and Analysis in Pharmaceutical Science: Validation, Process Controls, and Stability Paperback – 19 June 2019
Methodologies in Biosimilar Product Development
Design and Analysis of Bridging Studies
Elementary Bayesian Biostatistics
Encyclopedia of Biopharmaceutical Statistics, Second Edition
Statistical Design and Analysis of Stability Studies
Frailty Models in Survival Analysis
Statistics In the Pharmaceutical Industry
Translational Medicine: Strategies and Statistical Methods
Handbook of Adaptive Designs in Pharmaceutical and Clinical Development
Encyclopedia of Biopharmaceutical Statistics, Third Edition
Encyclopedia of Biopharmaceutical Statistics, Second Edition
Design and Analysis of Animal Studies in Pharmaceutical Development
Encyclopedia of Biopharmaceutical Statistics, Third Edition
Biosimilars: Design and Analysis of Follow-on Biologics
Statistics In the Pharmaceutical Industry
Controversial Statistical Issues in Clinical Trials
Innovative Statistics in Regulatory Science
Innovative Methods for Rare Disease Drug Development
Analytical Similarity Assessment in Biosimilar Product Development
Shein-Chung ChowDesign and Analysis of Bioavailability and Bioequivalence Studies, Third Edition BABE-Solution bundle version (Chapman & Hall/CRC Biostatistics Series) Chow, Shein-Chung and Liu, Jen-pei
Advanced Statistics in Regulatory Critical Clinical Initiatives (Chapman & Hall/CRC Biostatistics Series)
Multiple Testing Problems in Pharmaceutical Statistics (Chapman & Hall/CRC Biostatistics Series)
Biosimilar Drug Product Development (Drugs and the Pharmaceutical Sciences)
Encyclopedia of Biopharmaceutical Statistics - Four Volume Set [Hardcover] Chow, Shein-Chung Hardcover – 24 September 2018
Chung Jung One Korean Bidan Red Chili Pepper Flakes Fine Powder Gochugaru 500g (To Add Spice)
Innovative Methods for Rare Disease Drug Development (Chapman & Hall/CRC Biostatistics Series) Paperback – Import, 30 May 2022
Chung-shi Women's Comfort Step casual Lace-Up
Sample Size Calculations in Clinical Research (Chapman & Hall/CRC Biostatistics Series)
Design and Analysis of Animal Studies in Pharmaceutical Development: 1 (Chapman & Hall/CRC Biostatistics Series) Hardcover – 15 January 1998
Shein–Chung ChowDesign and Analysis of Clinical Trials: Concepts and Methodologies (Wiley Series in Probability and Statistics)
Handbook of Adaptive Designs in Pharmaceutical and Clinical Development
Advanced Linear Models: Theory and Applications: 141 (Statistics: A Series of Textbooks and Monographs)
Quantitative Methods for HIV/AIDS Research (Chapman & Hall/CRC Biostatistics Series)
Innovative Statistics in Regulatory Science (Chapman & Hall/CRC Biostatistics Series)
Analytical Similarity Assessment in Biosimilar Product Development [Hardcover] Chow, Shein-Chung Hardcover – 8 August 2018
Encyclopedia of Biopharmaceutical Statistics, Third Edition
Encyclopedia of Biopharmaceutical Statistics, Second Edition: Volume 1 Hardcover – 4 June 2003
chung shi Women's Sensomo Ii Brogues
chung shiMen's Sensomo I Brogues
Chung Shi Women's Duxfree Vancouver Women's, Sneaker, Brown, 36 EU
Methodologies in Biosimilar Product Development (Chapman & Hall/CRC Biostatistics Series) Hardcover – Import, 24 September 2021
Chung Shi Women's Comfort Step casual Lace-Up Black 9100215 5 UK








![[(Encyclopedia of Biopharmaceutical Statistics)] [ Edited by Shein-Chung Chow ] [May, 2010]](https://m.media-amazon.com/images/I/41wRKZ9msTL.jpg)












































![Encyclopedia of Biopharmaceutical Statistics - Four Volume Set [Hardcover] Chow, Shein-Chung Hardcover – 24 September 2018](https://m.media-amazon.com/images/I/51YHaWw-yhL.jpg)










![Analytical Similarity Assessment in Biosimilar Product Development [Hardcover] Chow, Shein-Chung Hardcover – 8 August 2018](https://m.media-amazon.com/images/I/41IQRELi1hL.jpg)






